Cellulose could qualify as Canada’s emblematic organic compound, the building block of the many different paper products that emerge from the country’s vast forests. It could also represent one of our most fundamental environmental challenges, since the tough bonds that characterize these molecules must be broken down with enzymes in harsh bulk solvents such as organometallic agents or strong acids that wind up in the outflow of facilities like pulp plants.
However, as McGill University Department of Chemistry professor Karine Auclair points out, nature has found a way to do the job without making such a mess. Fungi sitting on tree bark dissolve the cellulose found in that particular substrate merely by spitting out enzymes onto the surface, without the need for any kind of aqueous cocktail that scientists like her invariably associate with enzymatic activity.

Enzymes at work: fungi on tree bark show us how to break down cellulose without the need for bulk solvents.
“People use enzymes in dilute solutions all the time,” she explains. “This is the way things work, this is the way people assume things should work, and it has never been questioned. Now we can tell people: the way you’ve been doing enzymology is not always the best way.”
Auclair has discovered a better way with her first foray into the world of mechanochemistry, where physical interactions become the primary driver of reactive chemical processes. In collaboration with the laboratory of her departmental colleague Tomislav Friščić, who has been working in this field for several years, she and her colleagues started by subjecting microcrystalline cellulose to varying amounts of different cellulases, the hydrolytic enzymes that break down cellulose bonds. They reduced the mass fraction of these reagents to as little as 0.5% w/w and then aged them in liquid for as long as a week.
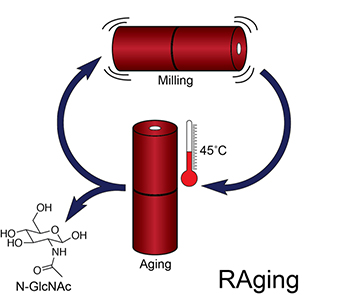
The resulting hydrolysis rates were far lower than might be found in a commercial processing operation using bulk solvents, but they jumped considerably when the samples were treated in a ball-milling jar, then left to age, with these two steps being repeated over an extended period. This technique, dubbed reactive aging, or RAging, calls for multiple iterations of five minutes of milling followed by 55 minutes of aging. After 12 cycles, conversion rates rose to between 50% and 100%.
“Here we’re showing that under mechanoenzymatic conditions we can get events to work more efficiently than in solution,” Auclair observes.
The findings appeared recently in Angewandte Chemie, where the authors describe the implications as nothing less than “a paradigm shift in enzymatic transformation of biomass and other recalcitrant substrates”. The applications go well beyond cleaning up the downstream output of pulp mills, as RAging could reduce the energy and waste necessary to deal with products such as sawdust, corn stover, and wheat chaff.
RAging is now being applied to break down chitin discarded during the processing of shellfish. Photo credit: J. P. Daniel Therien
Initially supported by a grant from NSERC’s Idea to Innovation program, which aims to accelerate the development of promising industrial technologies, Auclair has filed a patent on the process and entered into a partnership with GreenCentre Canada, which will help to scale up the method for commercial use.
At the same time, this successful experience with mechanochemistry has inspired her to continue exploring this approach to other areas that could benefit from the elimination of bulk solvents. RAging could prove to be equally effective in breaking down the massive amounts of chitin generated by shellfish plants as well as xylans put out by the forestry sector.
“What amazes me is how versatile this is,” she says. “This is something that I never expected would go that far and work so well.”
